The document control procedure of any company describes the control mechanisms to ensure that master documents are controlled with regards to their approval, revision, issue level, distribution and use.
The control of documents procedure helps to ensure that the company’s environmental, health, safety and quality management documentation needs are controlled at all times.
A good document control and management system helps to ensure:
- Document version control: All the documents related to Integrated Management System are properly reviewed and approved prior to issue hard copy or on electronic document management system / edms system.
- Controlled copy availability: Relevant controlled documents are available at the appropriate locations / projects.
- Document change control: Changes to controlled copy of all documents are properly authorized and indicated.
- Obsolete document disposal and archiving.
What is a Controlled Document?
A controlled document must meet the following conditions:
- It must be numbered according to the defined numbering scheme.
- It must be stamped “CONTROLLED DOCUMENT” on its title page to avoid its unauthorized copying and usage. (Un-controlled documents or copies do not contain any control stamp).
- It must be reviewed and approved before issue.
- Changes are authorized and controlled as a result of periodical review and revision as necessary.
- Current versions of relevant documents and data are available at all locations, where operations affecting the integrated management system are performed.
- Archival documents and data retained for legal or knowledge preservation purpose or both, are suitably identified.
Scope of document control procedure
The procedure applies to all documents related to Quality, Environmental and Occupational health & Safety Management System also known as integrated management system IMS, which can expand to any other system.
This procedure can cover all activities but not be limited to the following:
- Quality/ Environmental /Occupational Health & Safety Management System Manual
- Quality System procedures / Environmental System procedures & OHS system Procedures.
- Work Instructions / Method Statements
- Drawings
- Technical Submittals
- RFI’s
- External Correspondences
- Legal and Regulatory documents
- Tech documents (Standard/specifications, machinery manuals etc)
- Audit reports
- Quality Control Checklists
- Forms & reports
- Contractors
- Suppliers etc.
As per ISO 9001:2015 latest revision top management is overall responsible for implementation of document control procedure.
But generally quality head or manager is responsible for the implementation of this procedure.
Quality manager also working as management representative MR maintains the master list of documents indicating the revision status and distribution of all the documents under the documents control system.
Additionally for a project, QA/QC engineers and document controllers are responsible to maintain site documents in compliance with this procedure and corporate Electronic Data Management System.
Document Control Procedure
MR maintains an up to date copy of master list of company documents indicating the status and distribution of all the documents under the documents control system.
Coding Scheme for Forms, Registers, Lists, Plans and Technical Documents
Company has defined a numbering scheme for unique identification of forms, registers and lists etc. The format of the numbering scheme is as follows:
<Relevant Procedure Code> – <Type of Document><Serial No>
Example: IMSP-01-F1
Where “IMSP-01” is “Relevant Procedure code
“F” is the “Type” of the document i.e. Form, and “1” is the “Serial Number” of the form.
For method statements and submittals below format is used:
<Company Code><Department Code> – <Document Code> – <Sequence Number>
Example: COMPANY-FF-MST-01 is first method statement of firefighting department.
For drawings the numbering scheme is used as per the site requirements, if not available the above scheme can be used by adding the site specific terms including project name.
Example: COMPANY-EL-DWG-SD-GF-01 where SD stands for Shop Drawing & GF for Ground floor.
Most common types of controlled document codes are as follows:
|
Document Code |
Document Name |
| MST
ITP |
Inspection & Testing Plan |
|
ORG |
Organizational Chart |
|
RFI |
Request For Information |
| DWG
SD RCP AB GL |
Drawings Shop Drawing Reflected Ceiling Plan As Built Drawing General Layout |
|
SUB |
Technical Submittal |
|
EP |
Emergency Plan |
|
JD |
|
|
F |
Forms |
|
PD |
Project Documents |
|
PHSP |
|
| PQP | |
|
L |
List |
|
R |
Register |
Document Control Reference Numbers
Company has defined a numbering scheme for unique identification of letters/memos which can be used for both internal & external correspondences. The format of the numbering scheme is as follows:
<Company Code>/<Relevant Department Code>-<File Number>-<Job Code*><Serial No.>
Example: ABC/CVL-01/PR-01/001
ABC is…… Company code
CVL-01… is relevant department file code i.e. Civil Department file number 01
PR-01…. is Job Code unique for each project (Not applicable for all internal company departments)
001 is Serial Number of letters/memos in ascending order.
Some companies also add month or year in this document code scheme.
Details of some department name are as under which can be different for different type of companies:
|
Department Code |
Department Name | Department Code | Department Name |
|
AC |
Air Conditioning |
TD |
Tendering & Estimation |
|
EL |
Electrical |
QSHE |
|
| CVL | Civil |
ST |
Stores |
|
PL |
Plumbing |
CC |
Contracts & Claims |
| PR | Procurement | FM | Facility Management |
Procedure for Document Approval and Issue
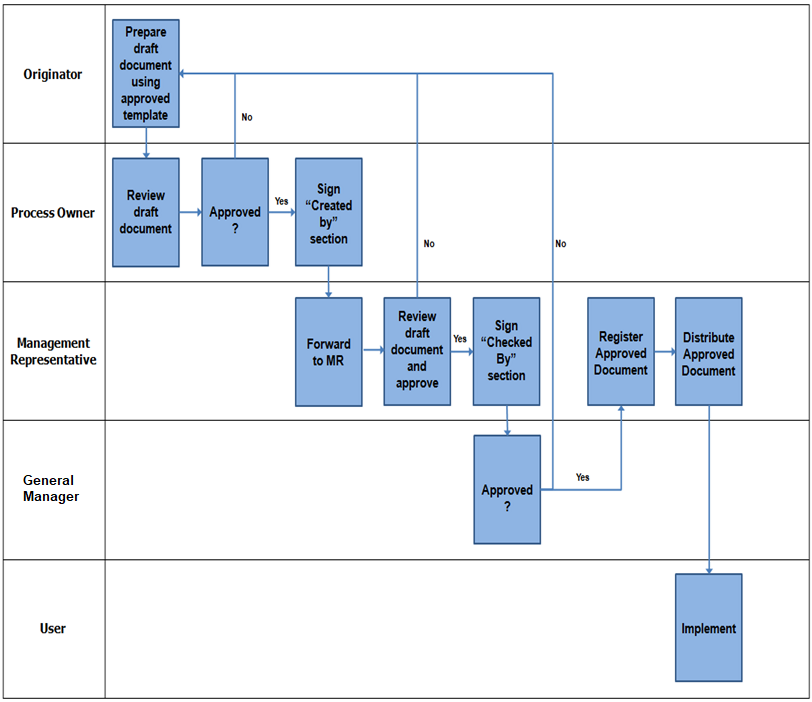
After preparation and numbering of documents, these are reviewed and approved by the competent authority as applicable in case of project or office related documents.
Following persons are authorized for reviewing and approving various IMS documents and sign the title cover page of the procedures.
| Type of Documents | Prepared By | Reviewed By | Approved By |
| IMS Manual | Quality Manager | General Manager | General Manager |
| IMS Procedure | Quality Manager | Department Heads | General Manager |
| Method Statement & ITP | QA/QC Engineer | QA/QC Manager | Project Manager |
| Risk Assessment | HSE Engineer | HSE Manager | Project Manager |
| Drawing | Draftsman | Project Engineer | Project Manager
Engineering Manager |
| Project HSE Plan | HSE Manager | Operation Manager / PM | General Manager |
| Project Quality Plan | Quality Manager | Operation Manager / PM | General Manager |
| Emergency Plan | HSE Manager | Operation Manager / PM | General Manager |
| Job Description | Department Heads | Operation Manager |
General Manager |
These approved documents like IMS manual & procedure are issued to the concerned departments/sections/sites.
Record of distribution is maintained on document distribution form if printed copies are distributed.
In case of distributing the scanned copies via emails no record of distribution is generated on prescribed form, instead email records can be used for reference.
Sincere efforts are always made to ensure that documents and data of activities, hazardous material data sheets, procedures and instructions are available and accessible to project personnel as and when required under routine and non routine conditions including emergencies.
External Documents Control
This is also important to identify and control the external documents. Following documents used in different departments are of external origin:
- Legislation and regulations issued by Government or other regulatory bodies i.e. codes and standards.
- Technical Documents (such as operational manuals, maintenance and technical manuals etc).
- Product standards / specifications / catalogs.
- Contracts
- Trade Licenses
- Management system standards
- Trade Certificates
These external origin documents need not to be numbered or approved by the competent authority.
External documents are stamped as “EXTERNAL DOCUMENTS” to avoid unauthorized copying and usage and same is included in master list of documents.
Each project and department maintains a list of external documents if any related to that department for traceability and retrieval.
Changes in Controlled Documents
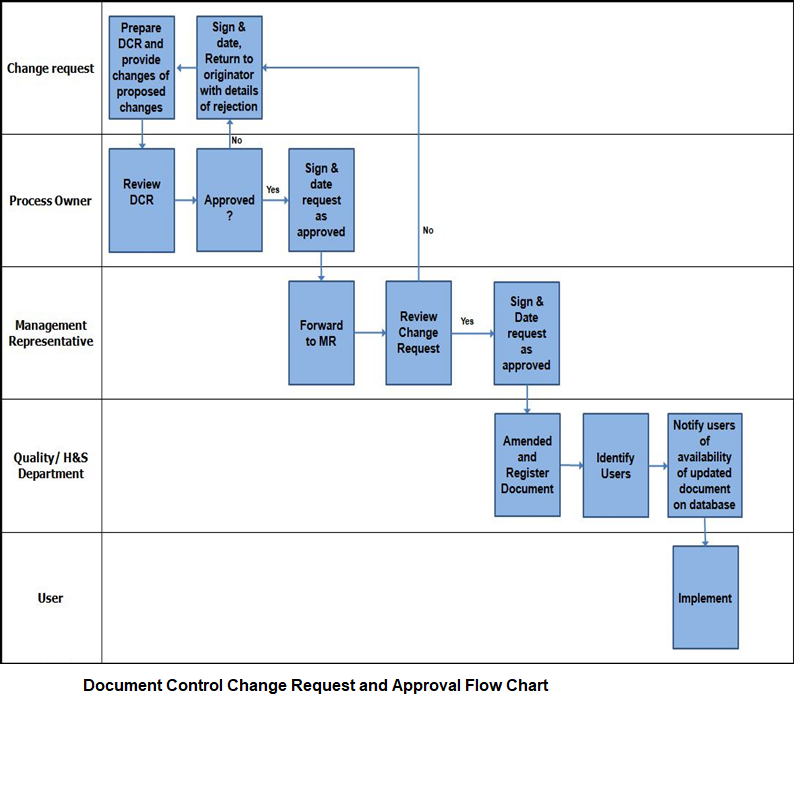
Any company persons who needs, can initiate changes in controlled documents by filling in documents change request (DCR) form.
The DCR is reviewed and approved by the competent authority as per review and approval procedure as mentioned above.
The revision number of the corresponding document is incremented on the relevant page and the revised document is finally approved by the concerned authority.
The revised document is then distributed to all concerned persons in the distribution list and the obsolete documents are retrieved.
Obsolete documents are either destroyed or stamped “OBSOLETE” for proper identification.
One copy of obsolete documents is maintained for record purpose. Quality manager or MR is responsible for controlling all these activities.
It is the responsibility of the heads of departments and divisions to ensure that any changes made to their documentation is flagged up to the MR.
Department head is also responsible to make sure that all documents held on the central database in EDMS are up to date and reflect the current work environment of the company.
Heads of departments shall review their documentation. For any changes that is required, a document change request shall be raised. Approval of this form shall be taken from the process owner & approved copy passed to the MR, who will review the changes, followed by updating & issuance of the new document.
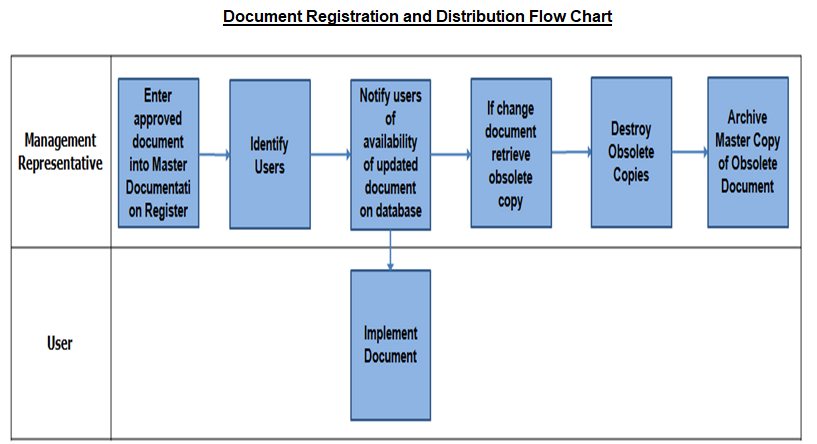
Controlled Copy Distribution & Administration
The administration of controlled documents is the responsibility of MR. The master list specifies the revision status of all the document numbers and titles of the controlled documents.
Each document is approved by the relevant person and document approval form is part of each controlled document as evidence.
This is the responsibility of MR to get the document distribution filled prior to issue of any document.
Person distributing the documents is responsible to ensure that documents are delivered to the concerned personnel and he maintains the record in the form of receiving signatures, fax reports or telephonic confirmations etc.
Approved controlled and uncontrolled documents are also maintained on EDMS system which is accessible to all employees of the company depending upon the access rights at different levels.
Archiving the Project Controlled Documents
Document controllers are responsible for archiving and sending to stores all the project related documents in accordance with the company policy. All the documents shall be filed and indexed with suitable records and tags for storage in the archiving center.
For construction projects, all the key project documents such as As-Built Drawings, Operations and Maintenance O&M Manual, Subcontractors contract agreements, invoices, snags & punch lists, authority approved documents and proof of approval and handover of project deliverables must be properly indexed.
Relevant Forms for Control of Document Procedure
- Master list of Documents & Forms
- External Documents List
- Document Approval Form
- Document Distribution
- Document Change Request
Flow Charts for Document Control Procedure
Preparation and Issue of new quality management system documentation
Document Registration and Distribution Flow Chart
Document Control Change Request and Approval Flow Chart
Discover more from Project Management 123
Subscribe to get the latest posts sent to your email.
One thought on “Document Control Procedure for Manual & Electronic Document Management System”
Comments are closed.